| Parameters | Description |
|---|---|
| Category of Drug | Acetazolamide is an Ophthalmological Preparation drug |
| Mechanism of Action | Acetazolamide causes inhibition of carbonic anhydrase in the CNS, which decreases carbon dioxide tension in the pulmonary alveoli, thus increasing arterial oxygen tension. Acetazolamide thus reduces intra-ocular pressure. |
| Indications | Acetazolamide is used 1. As an adjunct in the treatment of chronic open-angle glaucoma 2. Secondary glaucoma 3. As part of the pre-operative treatment of acute angle-closure glaucoma |
| Chemical Structure 3 | 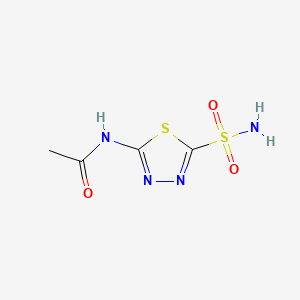 |
| IUPAC Name | N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide |
| Molecular details | Weight: 222.245 Chemical Formula- C4H6N4O3S2 |
| Pharmacokinetic properties 2 | Absorption-Not Available Volume of distribution- Not Available Protein binding- 98% Metabolism- Not Available Route of elimination- Not Available Half-life-3 to 9 hours |
| Well Known Pharmaceutical Brands | 1. Diamox- Sun 2. Iopar- FDC |
| Available dosage forms | 1. TABLET 2. CAPSULE  |
| Dose | Adult- 0.25 to 1 gm daily in divided doses |
| Contraindications | 1. Hypersensitivity to sulfonamides 2. Chronic angle-closure glaucoma 3. Hypokalaemia 4. Hyponatraemia 5. Hyperchloraemic acidosis 6. Renal impairment 7. Severe hepatic impairment 8. Renal hyperchloremic acidosis 9. Addison’s disease |
| Precautions | 1. Elderly 2. lactation 3. Diabetes Mellitus 4. Pulmonary obstruction 5. Monitor blood count and electrolytes if used for long periods 6. Interactions 7. Pregnancy |
| Adverse Effects | 1. Nausea 2. Vomiting 3. Diarrhoea 4. Taste disturbance 5. loss of appetite 6. Paraesthesia 7. Flushing 8. Headache 9. Dizziness 10. Fatigue 11. Irritability 12. Renal calculi 13. Blood disorders including agranulocytosis and thrombocytopenia 14. Rashes including Stevens- Johnson syndrome and toxic epidermal necrolysis |
| Pregnancy Category | C |
Other Ophthalmic preparation
- betaxolol
- Clonidine
- Latanoprost
- Physostigmine
- Pilocarpine
- Timolol

Pingback: Betaxolol- Indications, Dosage, Contraindications, ADRs - PharmaCampus
Pingback: Teva Pharma launch Brinzolamide Ophthalmic Susp > PharmaCampus