| Parameters | Description |
|---|---|
| Category of Drug | Gemcitabine is Immunosuppressives Drug |
| Mechanism of Action 1 | Gemcitabine inhibits thymidylate synthetase, leading to inhibition of DNA synthesis and cell death. |
| Indications 2 | Gemcitabine is used for 1. Pancreas cancer 2. Non-small cell lung cancer 3. Bladder cancer 4. Soft-tissue sarcoma 5. Metastatic breast cancer 6. Ovarian cancer |
| Chemical structure | 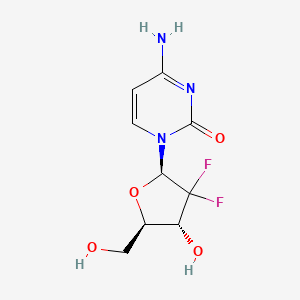 |
| Molecular details 3 | Molecular Formula: C9H11F2N3O4 Molecular Weight: 263.2 g/mol |
| 1. Absorption- 2-compartment model. 2. Volume of distribution- 50 L/m^2 3. Plasma protein binding is negligible (<10%) 4. Metabolism- Transformed via nucleoside kinases 5. Route of elimination- 92% to 98% of the dose excreted via urine 6. Half-life- 42 to 94 minutes | |
| Well Known Pharmaceutical Brands | 1. GEMCITE- ELI LILLY 2. CITAFINE- EMCURE 3. GEMITA- FRESENIUS KABI 4. MYGEM- MYLAN PHARMA 5. CYTOGEM- DRL |
| Available dosage forms | INJECTION |
| Dose | 1g/m2 body surface area for over 30 min once a week; for up to 7 weeks |
| Contraindications | 1. Pregnancy 2. Concurrent radical radiotherapy 3. Hypersensitivity 4. lactation |
| Precautions | 1. Gemcitabine is not recommended for patients who can have potentially curve surgery 2. There is insufficient evidence about its use for second-line treatment of pancreatic adenocarcinoma 3. Hepatic impairment 4. Renal impairment 5. Interactions |
| Adverse Effects | 1. Nausea 2. Vomiting 3. Oral mucositis 4. Hyperuricaemia 5. Bone marrow suppression 6. Alopecia 7. Thromboembolism 8. Flu-like syndrome 9. Edema 10. Thrombocythemia; 11. Somnolence |
| Pregnancy Category | D |