| Parameters | Description |
|---|---|
| Category of Drug | Amlodipine is an Antihypertensive Drugs. Read more: Which are the popular Medicines prescribed for Heart Disorders? |
| Chemical structure | 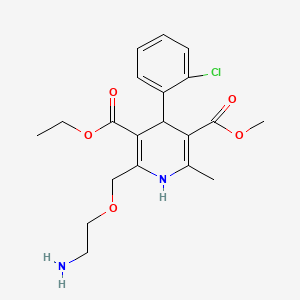 |
| IUPAC Name | 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate |
| Molecular details | Mol. formula- C20H25ClN2O5 Mol. weight- 408.9 g/mol |
| Mechanism of Action | It acts directly on the vascular smooth muscle and decreases the peripheral vascular resistance by inhibiting the influx of calcium ions into the vascular smooth muscle and cardiac muscle. This, in turn, causes a decrease in blood pressure. |
| Indications | Amlodipine is used for 1. Angina 2. Hypertension 3. Coronary artery disease |
| Well Known Pharmaceutical Brands | STAMLO- DRL AMLODAC- ZYDUS CADILA AMLOKIND- MANKIND AMLONG – MICRO LABS AMLOVAS- MACLEODS AMLOPRES- CIPLA STAMLO- DRL AMLOSAFE- ARISTO AMLOGARD- PFIZER AMTAS- INTAS |
| Available dosage forms | TABLETS |
| Dose | Initial dose: 5 mg orally once a day Maintenance dose: 5 to 10 mg orally once a day. Maximum dose: 10 mg/day |
| Contraindications | 1. Significant aortic stenosis 2. Sinoatrial node disease 3. Hypersensitivity to dihydropyridines 4. Cardiogenic shock 5. Unstable angina 6. Interactions |
| Precautions | 1. Hypotension 2. Myocardial infarction 3. Impaired renal funcon sick-sinus syndrome 4. Severe ventricular dysfunction 5. Hypertrophic cardiomyopathy 6. Severe aortic stenosis 7. Elderly 8. Children 9. Pregnancy |
| Adverse Effects | 1. Arrhythmias 2. Postural hypotension 3. Dizziness 4. Ankle edema 5. Hypoesthesia 6. Flatulence 7. Asthenia 8. Muscle cramps 9. Conduction system delay 10. System delay 11. Abdominal pain 12. Headache 13. Sleep disturbances |
| Pregnancy Category | C |
Referernces:


Pingback: Which are the popular Medicines prescribed for Heart Disorders? - PharmaCampus