
Accuracy: Accuracy is the extent to which a value from a test reflects or agrees with the reference value of the analyte being tested, measured statistically by standard deviations. In general, accurate test methods measures what it is supposed to measure.

Validity: It refers to the ability of a device to measure what it intends to measure. It is not an intrinsic test property and is an essential element of medical diagnosis.

Reliability: It is the ability to produce reproducible results. Reliability is a prerequisite for the validity of a test that consistently produces true measurements.

Sensitivity: It is used to describe how well a test can detect a specific disease or condition in patients who actually have them. It also refers to the way the body
reacts to the environment, drugs, chemicals, and other substances.

Precision: It is a measure of test or assay reproducibility that is capable of producing the same results when performed on the same specimen under the same condition. The amount of random variation is less when the test method is precise.

Addiction: It is a biopsychological disorder that is characterized by compulsive engagement in activities that help to achieve the desired effects, such as alcohol
an intoxication to attain euphoria despite the harm and adverse effects to self and others.

Affinity: It is a measure of tightness with which a drug binds to the receptor. It is also defined as the probability that a drug molecule will bind to an available receptor at any given instant of time.

Agonist: Agonists are drugs that bind to and activate receptors to elicit a pharmacological or biological response. It has good affinity and efficacy.
Hormones and neurotransmitters are also known as endogenous agonists.

Partial Agonist:
These bind to and activate a given receptor but only have partial efficacy even at maximal receptor occupancy. It has both agonistic and antagonistic activities and is often used by physicians to elicit a desired submaximal response in the patients. Eg. Buspirone
Full agonist:
These drugs bind to and activate the receptors to elicit the maximal response that an agonist is capable of producing. Eg. Isoproterenol
acts as a full agonist at beta receptors by mimicking the action of adrenaline.

Inverse Agonist
It binds to the same receptor as that of the agonist but
induces a pharmacological response that is opposite to that of the agonist. An inverse agonist has negative efficacy. Eg. Naloxone and naltrexone act as partial
inverse agonists at mu-opioid receptors.

Allergic Response:
It is a hypersensitive immune response to a substance (food, chemicals, drugs or pollen grains) that is normally harmless and would not cause an immune response in everyone. Itching, inflammation and tissue injury are some of the well-known allergic responses.
Analgesic:
Analgesics are medicines that help to produce analgesia or provide relief from pain. It is also known as pain relievers or pain killers and act at the
peripheral and central nervous system. Eg. Paracetamol, codeine etc.
Anesthetic:
It is a drug that induces anesthesia or temporary loss of sensation or awareness. General anesthetics cause a reversible loss of consciousness while local anesthetics cause a reversible loss of sensation in a restricted region without affecting consciousness.

Antagonism:
Antagonists are drugs that bind to and block the receptors to dampen a pharmacological or biological response. It has good affinity but no efficacy. It will inhibit the binding of the agonists or inverse agonists with the receptors and is also known as blockers.
AUC (Area under the curve): It is the total integrated area under the plasma drug concentration versus time curve and expresses the total amount of drug
that reaches the systemic circulation following its administration. Drugs administered via intravenous route has higher AUC than the oral drugs.

Bioassay or Biological assay: It is an analytical method used to determine the potency or concentration of an active principle in the unit quantity of preparation by measuring its biological response in living tissues. It compares the activity of the test substance with that of the standard.

Bioavailability:
It is also known as biological availability and is the rate and extent (amount) of absorption of the unchanged form of the drug from the dosage form at the site of administration. Medications administered intravenously show 100 % bioavailability.
Biopharmaceutics:
It is the study of the factors influencing the rate and amount of drug that reaches the systemic circulation and the use of this information to optimize the therapeutic efficacy of the drug products.
Biotransformation:
It is also known as metabolism and refers to the chemical alteration of chemicals like nutrients, amino acids, toxins, and drugs in the body. This helps to convert the non-polar and hydrophobic compounds into polar and hydrophilic compounds to facilitate its excretion from the body.

Blind Experiment:
It is a type of experiment in which the experimenter does not know which treatment is given to the subject. This helps to eliminate the bias that might be introduced by the researcher. In pharmacological studies, both patients and experimenters are blinded and this is called a double-blind experiment.
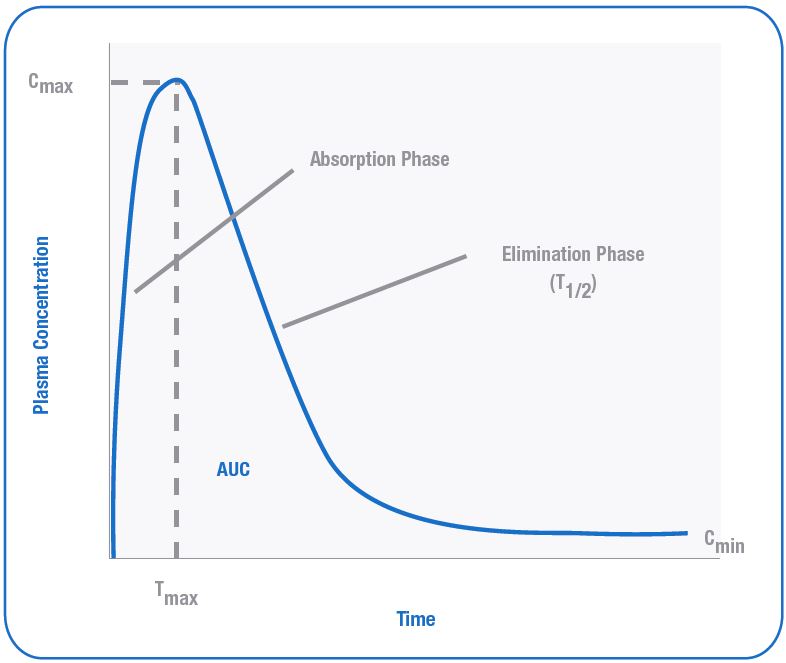
Cmax:
C max is the peak plasma concentration that a drug
achieves in a specified compartment after initial administration and prior to the administration of the second dose.
Cmin:
C min is the minimum blood plasma concentration of the drug prior to the administration of the second dose. It must be above the minimum inhibitory concentration to produce therapeutic effects.

Ceiling:
The ceiling effect is a phenomenon in which a drug reaches a maximum effect, such that a further increase in drug dosage does not increase the effectiveness. Eg. Nalbuphine produces a ceiling effect. Increasing the dose of nalbuphine beyond a certain value leads to smaller gains in pain relief.

Chemotherapy:
It is a type of cancer treatment that uses chemotherapeutic agents or anti-cancer drugs as a part of standardized therapy. It is given with
either a curative intent or to prolong the life of the patient.
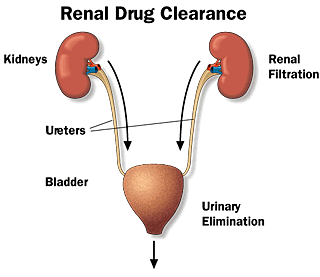
Clearance: It is the hypothetical volume of body fluids containing drug from which the drug is removed or cleared completely in a specific period of time. It is measured in L/hr or mL/min and reflects the rate of drug elimination divided by the plasma concentration.

Clinical Therapeutic Index: It is a quantitative measurement of the relative safety of the drug. It refers to the ratio of the dose of the drug that causes adverse effects (TD50) to the dose that leads to the desired pharmacological effect (ED50).
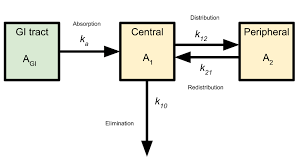
Compartment(s): It is a fictitious or virtual tissue or group of tissues that have similar drug distribution characteristics (similar blood flow and affinity to the drug). These are mathematical constructs and do not correspond to the fluid volume of the body that is defined- physiologically and anatomically.
Compliance: It describes the degree to which a patient correctly follows medical advice. It commonly refers to medication or drug compliance but may also be applicable to other self-directed exercises and therapy sessions.

Cross-Over Experiment: Cross-over experiments are experiments in which each subject acts as his/her own control as they take each of the treatments under investigation on different occasions. It is particularly efficient in the cases of large inter-individual variation.
Cross-Tolerance: It is a phenomenon that occurs when the tolerance to the effects of certain drugs produces tolerance to another drug. This is especially seen when the two drugs have similar functions or effects. Eg. Cross-tolerance between amphetamine and caffeine.

CT Index: It is a measure of the drug potency that is calculated by multiplying the concentration (C) of a therapeutic agent administered to the body to produce a specific response with the duration of application (T) applied to elicit the effect.

Dependence:
It is synonymous with addiction and habituation and refers to the somatic state that is attained following chronic administration of certain drugs. In this state, the individual becomes dependent on the drug and sudden discontinuation of this drug produces withdrawal symptoms that may even prove to be fatal.

Desensitization: It refers to the reduction in the subject’s response following repeated use of the drug. Desensitization occurs when the individual receptors become less responsive to the drug post its repeated administration.
Disintegration Time: It is the time required for the dosage form to break up into granules of specified size under carefully specified test conditions. It is very important in the case of solid dosage forms like tablets and capsules and is directly related to the amount of binder present in the solid dosage form.

Dissolution Time:
It is the time required for the given amount or fraction of the drug to solubilize in a given solvent, i.e., mass transfer from the solid surface to the liquid phase.
Dosage Form:
It refers to pharmaceutical drug products in the form in which they are marketed for use, with a specific mixture of active ingredients and excipients, in a particular configuration, and apportioned in a particular dose.

Dose:
It is the measured quantity of a therapeutic agent that is to be taken at a specific time. Each dose corresponds to a single unit.

Dose-Duration Curve:
It is a curve describing the relationship between the dose and the duration of the drug effect. It is used to infer the pharmacokinetic and pharmacodynamic properties of the drug including elimination half-life and threshold dose. It always has a positive slope.
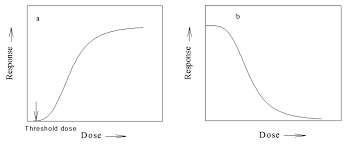
Dose-Effect Curve: This curve correlates the effect produced with the dose administered. It is continuous, monotonic, and always approaches some maximum value as an asymptote.
Drug:
It is any substance that causes a change in the organism’s physiology or psychology on consumption and is used in the treatment, cure, prevention or diagnosis of a disease. It can also be used to enhance the physical or mental well-being of the individual.

Drug Abuse:
It refers to the use of a certain drug for the purpose of creating pleasurable effects on the brain. It is also defined as the use of illegal drugs or the use of prescription and over-the-counter drugs for purposes other than those for which they are meant to be used or in excessive amounts.

Drug Dependence:
It is also known as substance dependence and refers to an adaptive state that develops from repeated drug administration which results in withdrawal upon cessation of drug use.

Dummy:
It is a counterfeit object used in the experimental investigation of the drug effects and is rendered to be biologically inert. It is used to get an unbiased assessment of the result of a pharmacological experiment.

EC50:
EC50 or Half maximal effective concentration refers to the concentration of the drug, antibody or toxicant (agonist) which induces a response that is halfway between the baseline and maximum after a specified exposure time. It can also be defined as the concentration required to obtain 50 % effect.

ED50:
It is the median effective dose and is defined as the dose of the drug that produces 50% of the maximal response to that drug. It is used when the measurements are taken in-vivo.

Effective drug:
A drug is considered to be effective if it achieves its intended effect in the usual clinical setting. It is evaluated through observational studies of real practice.

Efficacy:
It is the maximum response achievable from the applied or dosed agent in research and clinical settings. It is independent of potency or affinity but is related to the intrinsic activity.

Equipotent
Two drugs are said to be equipotent if they are equally capable of producing a pharmacological effect of specified intensity. Drugs with the same intrinsic activities and ceiling effects are called equipotent.
Equivalence:
Pharmaceutical equivalence means that the two products have the same active ingredients in the same dosage form and are intended for the same route of administration. Biological equivalence refers to the chemical equivalents which when administered in the same amount will produce the same bioavailability.

First-Order Kinetics: It occurs when a constant proportion of the drug is eliminated per unit time. It is a concentration-dependent process in which the clearance is faster at higher concentrations.

First Pass Effect:
It is a phenomenon of drug metabolism whereby the concentration of a drug, specifically when administered orally, is greatly reduced in the liver before reaching the systemic circulation. It is also known as pre-systemic metabolism.
Food and Drug Administration (F.D.A.):
The FDA is a federal agency of the United States Department of Health and Human Services that is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, medications and veterinary products among others.

Generic Drugs:
It is a pharmaceutical drug that contains the same active ingredient as the drug that was originally protected by chemical patents. These are allowed for sale after the patents of the original products expires. The pharmacological effects of the generic drugs and the patented products are the same.

Habituation:
It is the process of forming a habit and refers to the psychological dependence on the continued use of a drug to maintain a sense of well-being which can result in addiction. Tolerance to the effect of the drug is acquired through its continued use.
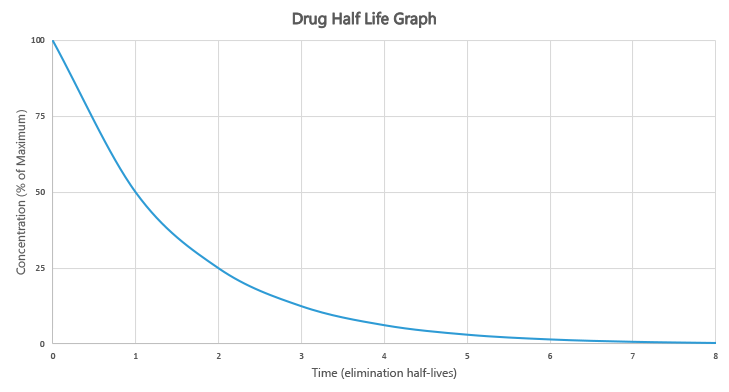
Half-Life:
It is the time required for the concentration or amount of the drug in the body to be reduced by one-half of its steady-state. It helps to determine the drug dose and frequency of administration.

Harrison Act:
The Harrison Narcotics Tax Act was a United States federal law that regulated and taxed the production, importation and distribution of opiated and coca products. It was approved in 1914 and enacted in 1915.

Hazardous drugs
In pharmacology, hazardous drugs are drugs that are known to cause harm, which may or may not include genotoxicity, carcinogenicity, teratogenicity, fertility impairment, or serious organ or other toxic manifestations at lower doses in humans or experimental animals.

Hypersensitivity:
It is an exaggerated immune response to a specific antigen or drug. Hypersensitivity reactions may be damaging, uncomfortable, or occasionally fatal and are due to the pre-sensitized immune state of the host.

Hypnotic:
It is also known as soporific drugs or sleeping pills and are a class of psychoactive drugs whose primary function is to induce sleep. It is used for the treatment of insomnia or for surgical anesthesia and is related to sedatives.
Idiosyncratic Response:
It is also known as type B adverse drug reactions that occur rarely and unpredictable among the population. Eg. Development of rashes following ampicillin administration in individual with infectious mononucleosis.
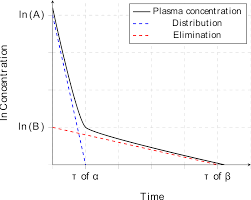
Infusion Kinetics:
Infusion in drug administration refers to the continuous flow of drug solution into the bloodstream over a relatively long period of time. It is used to maintain a steady plasma concentration of the drug and is directly proportional to the rate of drug administration.

Intrinsic Efficacy (or Intrinsic Activity):
It is the measure of the ability of a drug that is bound to the receptor to generate an activating stimulus and produce a change in the cellular activity. It refers to the relative ability of the drug-receptor complex to produce a maximum functional response.
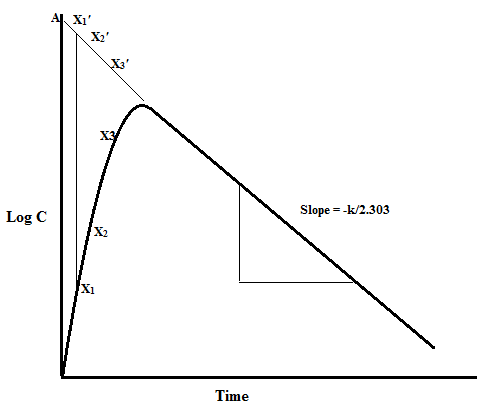
ka:
- The absorption rate constant (Ka) is a pharmacokinetic constant that is used to describe the rate at which a drug enters into the system. It is expressed in time-1. It is related to the biological half-life as follows:
Ka = ln/t1/2
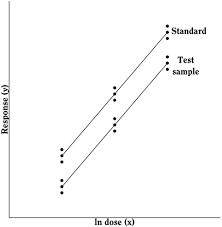
Metameter:
It is a quantity derived from a measurement used in evaluating biological tests. It facilitates statistical summary and analysis of data. Eg. Metameters of dose includes “milligrams”, “moles”, “log milligrams”, etc.

kel:
The elimination rate constant (Ke) is a pharmacokinetic constant that is used to describe the rate at which the drug is removed from the human system. It is equivalent to the fraction of the drug that is removed per unit time measured at any particular instant and has the unit of time-1.

Latent Period or Latency:
It is the period between infection with a microorganism and the onset of symptoms or between the exposure of radiation and appearance of cancer.

Loading Dose:
It is an initial higher dose of a drug that is given at the beginning of a course of treatment before dropping down to a lower maintenance dose. Drugs with long systemic half-life require a low loading dose.
Maintenance Dose:
It is the dose at which the maintenance rate of drug administration is equal to the rate of drug elimination at a steady state. It is calculated from the desired peak concentration multiplied by the clearance rate.

Median Effective Dose:
It is the dose or concentration of the drug that produces a biological response. It is generally defined as the lowest dose level of a pharmaceutical product that provides a clinically significant response in average efficacy.
Multiple Dose Regimens:
It refers to the administration of a successive dosage of a drug in which the drug is administered frequently with constant dose interval. It aims at maintaining the plasma drug concentration within the therapeutic range and to produce the maximum therapeutic effect.
Narcotic:
It refers to any psychoactive compound with sleep-inducing properties and euphoric properties. It is also defined as an addictive drug that reduces pain, induces sleep and alters mood and behavior. Eg. Morphine, codeine
National Formulary (N.F.):
It is an official publication that contains the standards of purity, methods of assay of some drugs, formulae, methods of manufacture of various pharmaceutical preparations, list of standard excipients, botanicals and other similar products. It was formerly published by the American Pharmaceutical Association.

Negative Control Drug or Negative Control Procedure:
The negative control drug used in pharmacological experiment lacks the active ingredient that elicits the biological response. It helps to prevent erroneous conclusions about the apparent activity of the test preparation.
Parameter:
It is an element of the experiment that can be varied but which the experimenter tries to control or maintain constant during the course of a specific experiment, while intentionally altering the independent variable and observing the changes on the dependent variable.

Pharmacodynamics:
It is a branch of pharmacology that deals with the biochemical and physiological effects of the drug on the body. It relates the response produced to the concentration of drug in the body.

Pharmacogenetics:
It is the branch of pharmacology that analyzes how the genetic makeup of an individual affects his/her response to the drugs. It deals with the influence of acquired and inherited genetic variation on drug response in patients by correlating gene expression with pharmacokinetics and pharmacodynamics.

Pharmacokinetics:
It is the study of the time course of a drug’s absorption, distribution, metabolism and excretion and its relationship with the toxic and therapeutic effects produced by the drug. It is simply defined as the study of what the body dose to the drug.

Pharmacology:
It is a branch of medicine and pharmaceutical sciences that is concerned with the study of the uses, effects, and mechanisms of action of drugs. The origin, composition, pharmacokinetics, therapeutic use, and toxicology of the drug are studied under this.

Placebo:
Placebos are substances that are made to resemble drugs but do not contain the active ingredient. It has no inherent pertinent pharmacologic activity but is rendered effective by the psychological suggestion given during administration.

Positive Control Drug:
It is a drug preparation incorporated into an experiment with the intention that it has effects on the experimental system qualitatively similar to those expected of the independent variable. It is used for validating the experimental system.

Potency:
It is a measure of the drug activity expressed in terms of the amount required to produce an effect of the required intensity. Highly potent drug evokes a given response at low concentrations while a drug of lower potency evokes the same response at higher concentrations.
Potentiation:
It is the interaction of two or more drugs or agents resulting in a pharmacological response that is greater than the sum of the individual responses of each drug or agent. Eg. Combination of sedative drugs with alcohol.
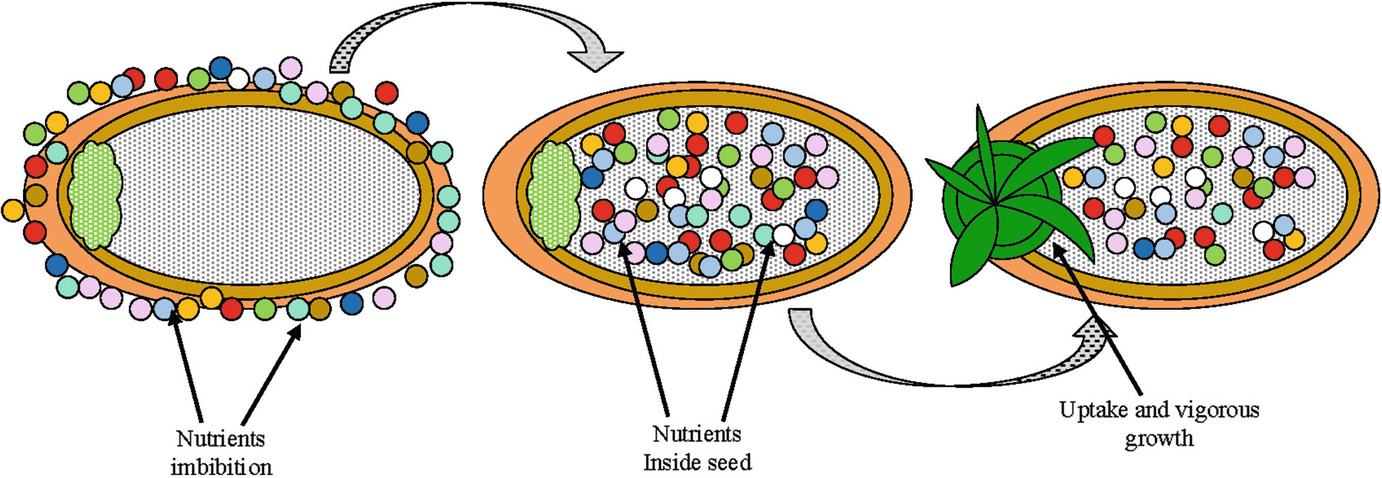
Priming Dose:
It is also known as the loading dose. It is defined as an initial higher dose of a drug that is given at the beginning of a course of treatment before dropping down to a lower maintenance dose. Drugs with long systemic half-life require a low loading dose.
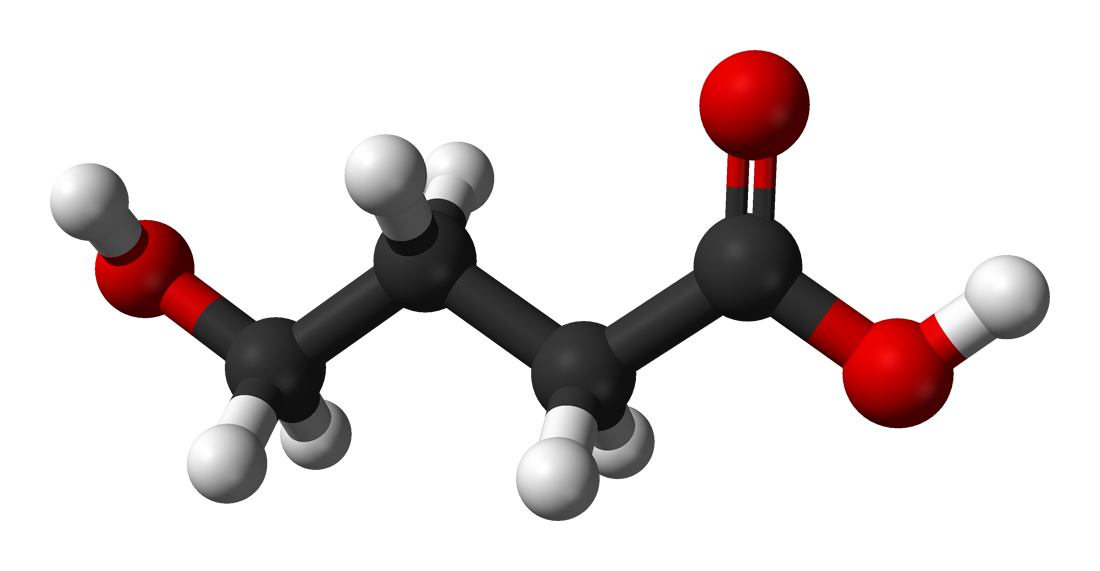
Prodrug:
It is a medication or compound that, after administration, is metabolized into a pharmacologically active drug. It is a precursor of the drug and is used to alter the pharmacokinetic properties of the drug. Eg. Sulfasalazine

Receptors:
These are chemical structures composed of proteins that bind to the ligands to elicit a physiological response. These proteins recognize and respond to endogenous chemical signals that are integrated into biological systems.

Reference Standard:
A pharmaceutical reference standard is a highly characterized material suitable to test the identity, strength, quality, and purity of substances for pharmaceutical use and medicinal products

Risk:
It is the likelihood that harm will result from exposure to a hazard. It implies future uncertainty of the effects of activity with respect to something that humans value, often focusing on negative and undesirable consequences.
Selectivity:
It is the degree to which a drug acts on a given site relative to other sites. It refers to the drug’s ability to preferentially produce a particular effect and is related to the structural specificity of drug binding to receptors.

Side Effects:
It is an effect that may be therapeutic or adverse and is secondary to the one intended. It predominantly refers to the adverse effects that are either harmful or unpleasant to the patient.

Spare Receptors:
Spare receptors are present when an agonist induces a maximum response while occupying less than 100 % of the available receptors. It reflects a circumstance in which the maximum effect produced by an agonist is limited by some factor other than the number of activated receptors.

Specificity:
It is a measure of the receptor’s ability to respond to a single ligand. A drug of good specificity of action will increase or decrease a specific function of a given cell type. Eg. Atropine is specific in blocking the actions of acetylcholine.

Supersensitivity:
It refers to the increased responsiveness or reactivity to a neurotransmitter or drug especially in a structure deprived of ita normal nerve supply (denervation). It can also be induced due to the administration of some other drug.

Synergy:
Drug synergy is the interaction of two or more drugs to produce a total effect that is greater than the sum of the individual effects of each drug. It can be either beneficial or harmful. Eg. Enhanced liver injury is produced due to synergism between carbon tetrachloride and ethanol.
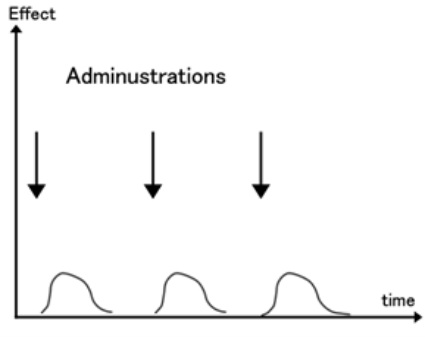
Tachyphylaxis:
It refers to an acute, sudden decrease in response to a drug after its administration; i.e. a rapid and short-term onset of drug tolerance. It can occur after an initial dose or after a series of small doses. Increasing the dose of the drug might be able to restore the original response.

Therapeutic Index:
- It is a quantitative measurement of the relative safety of the drug. It refers to the ratio of the dose of a drug that causes adverse effects (TD50) to the dose that leads to the desired pharmacological effect (ED50).
Therapeutic index = TD50/ED50

Therapeutics:
It is a branch of medicine that deals with the treatment of disease and the action of remedial agents. It can also refer to the therapy and drug used to remediate a health problem following diagnosis.

Threshold Dose:
It is the minimum dose of ionizing radiation, drug, or chemical that will produce a detectable degree of any therapeutic effect.

Time-Concentration Curve:
It is the graphical representation of the relationship between the concentration of dose of the drug and its latency or latent period. It is usually hyperbolic in nature and is characteristic for each drug.

Tolerance:
Drug tolerance is a pharmacological concept that is used to describe the patient’s reduced reaction to a drug following its repeated use. Increasing its dosage may re-amplify the drug’s effects but this may also accelerate tolerance and reduce the drug’s effects

Toxic Effects:
Toxic effects are responses to a drug that is harmful to the health of the individual. It refers to the adverse effects produced by poisons and can range from mild symptoms like headaches or nausea to severe symptoms like coma, convulsions, and death.

Toxicology:
It is a branch of science that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnosing and treating exposures to toxins and toxicants. It is concerned with the nature, effects and detection of poisons in living beings.

Two-state Model:
It is a simple linear model used to describe the interaction between a ligand and its receptor. This model proposes that ligand binding results in a change in receptor state from an inactive to an active state based on the receptor’s conformation

United States Pharmacopoeia (U.S.P.):
The U.S.P is a pharmacopoeia for the United States published annually by the United States Pharmacopeial Convention. It is published in a combined volume with the national formulary and establishes written and physical standards for medicines, food ingredients, dietary supplement products and ingredients.
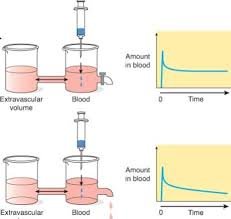
The volume of Distribution:
- It is a theoretical volume that would be necessary to contain the total amount of an administered drug at the same concentration as that observed in the blood plasma. It is also known as the apparent volume of distribution (Vd) and is calculated as follows:
Vd = Total amount of drug in the body/drug concentration in blood plasma

Zero-Order Kinetics:
These are reactions in which the rate of elimination of the drug is independent of the concentration of the drug. The amount of drug eliminated per unit time decreases linearly with time. Eg. Phenytoin, warfarin and ethanol follow zero order kinetics.
REFERENCES:
- WHO-GLOSSARY OF TERMS
- Glossary of Terms
- Pharmacology

It is very elaboration contents and much useful for a Pharma, medical and nursing peoples.
Very interseting and knoweldgable 👍👍
Nice for a glance,while in hurry to exam
Very useful for Academicians and Researchers in the Medical and Para Medical field.
Much useful for Academicians and Researchers in the Medical and Para Medical field.
Pingback: Pharmaceutical Content Writing: How can I learn this skill? - PharmaCampus
Pingback: What is an API or Active Pharmaceutical Ingredient? > PharmaCampus