Use of AstraZeneca’s COVID – 19 vaccine suspended in Ireland over the fear of developing blood clots.
The Health Ministry of Ireland announced its decision to temporarily stop using the AstraZeneca COVID vaccine from the morning of 14 March 2021. This precaution has been taken due to reported blood clots in adults after receiving the shots in Norway. 5
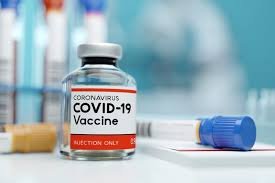
Ireland’s National Immunization Advisory Committee ( NIAC ) recommended the temporary suspension of this vaccine’s rollout. This is claimed to a “ precautionary principle “ that was put into effect. This decision was made as the Norwegian Medicines Agency received four new reports of serious blood clotting events in adults following vaccination.
It is yet to be concluded if there is any connection between the blood clot cases and AstraZeneca’s vaccine. But the action has been taken “ pending receipt of further information “.
The NIAC plans to meet on 14 March 2021 for discussing this matter and issuing another statement.
As of 10 March 2021, over 570,000 doses of coronavirus vaccines have been administered in Ireland. Among these, a total of 109,000 doses have been manufactured by AstraZeneca.
AstraZeneca COVID vaccine: What the company has to say?
AstraZeneca has claimed that there is no evidence for the increased risk of blood clot conditions based on the vaccine’s safety data. This data was obtained from over 17 million doses of the vaccine that has been administered to date.
It also added that the reported number of these events following vaccination was less. This was in comparison to the number that would occur naturally in the unvaccinated population.

Ireland is a country that has a population of over five million people. Over 4,534 people have succumbed to COVID – 19 as per the latest official figures.
The surge of cases has put the country in its third lockdown. This has also made the country the world’s most infectious nation in early January 2021.
The shortage of the vaccine combined with the sluggish nationwide rollout has put the country under great pressure. On 11 March 2021, the country’s health minister stated that AstraZeneca was repeatedly changing its delivery schedules at the last minute. Furthermore, it claimed that the pharma company often revised down the volumes it will deliver every time. The health minister also expressed his discontent and frustration over this matter.
- Pharma News – 23.01.2023 to 29.01.2023
 Mankind Pharma invests in Actimed Therapeutics to support the latter’s development of treatment for cancer… Read more: Pharma News – 23.01.2023 to 29.01.2023
Mankind Pharma invests in Actimed Therapeutics to support the latter’s development of treatment for cancer… Read more: Pharma News – 23.01.2023 to 29.01.2023 - Lupin issues nationwide recall of Clobetasol propionate cream.
 On 4 February 2023, Lupin announced the recall of Clobetasol propionate cream over quality issues.… Read more: Lupin issues nationwide recall of Clobetasol propionate cream.
On 4 February 2023, Lupin announced the recall of Clobetasol propionate cream over quality issues.… Read more: Lupin issues nationwide recall of Clobetasol propionate cream. - Global Pharma Healthcare recalls Artificial Tears Lubricant eye drops.
 The USFDA[1] has revealed that Global Pharma Healthcare recalls all the batches of artificial tears… Read more: Global Pharma Healthcare recalls Artificial Tears Lubricant eye drops.
The USFDA[1] has revealed that Global Pharma Healthcare recalls all the batches of artificial tears… Read more: Global Pharma Healthcare recalls Artificial Tears Lubricant eye drops. - Milestones in Cancer Research and DiscoveryCancer research and discovery is the process of uncovering new information about cancer and how… Read more: Milestones in Cancer Research and Discovery
- OAI notice for Torrent Pharma Indrad facility
 USFDA issues OAI notice for Torrent Pharma’s Indrad facility in Gujarat.In response to this notice,… Read more: OAI notice for Torrent Pharma Indrad facility
USFDA issues OAI notice for Torrent Pharma’s Indrad facility in Gujarat.In response to this notice,… Read more: OAI notice for Torrent Pharma Indrad facility - Cancer Cachexia: Mankind Pharma’s association with Actimed Therapeutics
 On 23 January 2023, Mankind Pharma announced its move to invest in the UK-based clinical-stage… Read more: Cancer Cachexia: Mankind Pharma’s association with Actimed Therapeutics
On 23 January 2023, Mankind Pharma announced its move to invest in the UK-based clinical-stage… Read more: Cancer Cachexia: Mankind Pharma’s association with Actimed Therapeutics

