Implantable drug delivery systems – Implants and Osmotic pumps:
Implants are sterile solid masses intended for implantation into the body by a minor surgical incision or injection through a large-bore needle. They are often placed in convenient yet inconspicuous regions under the skin, especially in the subcutaneous or intramuscular tissue. With limited drug loading capacity, they can store only a small concentration of highly purified drugs employing compression or molding or extrusion. These devices are often used to deliver insulin, steroids, analgesics, total parenteral nutrition, and heparin in patients. Let us discuss Implants and Osmotic pumps.
Implantable drug delivery devices are often classified into two categories, namely :
- Passive polymeric implants
- Active or dynamic polymeric implants
Passive polymeric implants

Passive polymeric implants – These implants do not have any moving parts and rely on passive diffusion to deliver drugs. In these devices, the drug is packed within a biocompatible polymer material. Based on the polymer’s nature, they are further subdivided into: non-degradable and degradable.
- Non – biodegradable polymeric implants – Silicones, polyurethanes, polyacrylates, and polyethylene vinyl acetate are commonly used polymers while in these implants. The drug in these implants can either be homogeneously dispersed in the polymeric matrix or surrounded by the permeable non – biodegradable membrane. These devices are often used for contraceptive purposes but need to be removed after the treatment.
- Biodegradable polymeric implants – These devices are made of polymers or copolymers that break down into smaller fragments and get excreted or absorbed by the body. The polymers commonly used in this case are polycaprolactone, polylactic acid, or polylactic – co – glycolic acid. Owing to their biodegradation, these implants need not be extracted after the treatment period. However, they are complex to produce as the polymers used are limited. The rate of degradation of the device is also not constant as it varies with each patient, and hence this is also a cause of concern while using biodegradable implants.
Active polymeric implants

Active polymeric implants – These implants have a higher degree of drug release control but are expensive to develop. It mainly includes pump-type implants such as osmotic pumps. The basic characteristic that distinguishes a pump from other controlled-release systems is that the primary driving force for delivering the drug by a pump is the pressure difference generated by pressurizing a drug reservoir.
This can be achieved by either osmotic action or by direct mechanical actuation. However, in other controlled–release systems, the primary force is the drug delivery system’s concentration difference in the drug delivery system and the surrounding tissue.
Osmotic pumps

Osmotic pumps – This is a type of controlled drug delivery in which the drug release occurs due to the osmotic pressure. Osmotic pumps are easy to formulate and simple to operate. Thereby, they improve patient compliance, prolong the drug’s therapeutic effect, and reduce the dosing frequency. Owing to these reasons, they offer an attractive alternative to other conventional dosage forms.
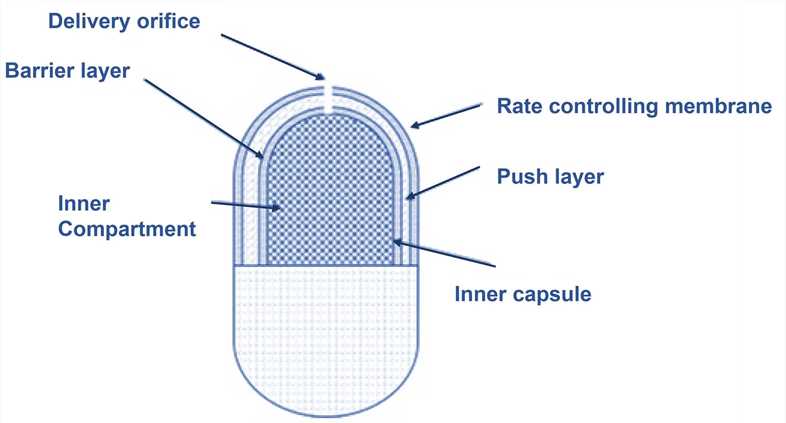
These are often administered orally and consist of a compressed tablet core with a semi–permeable membrane coating. This coating has one or more orifices through which the drug is released over a period of time. The central core comprises the drug with an osmotic agent and a water-swellable polymer. As the osmotic pump gets exposed to the aqueous environment, the core begins to adsorb water, which causes the polymer to swell and expand in volume. As a result of these changes, the drug solution or suspension is pushed out via the orifice, and the drug is released in a controlled manner.
Components of osmotic pumps :

Semipermeable membrane and polymers in drug matrix –The commonly employed semipermeable polymer for the preparation of osmotic pumps are cellulose acetate. A mixture of hydrophilic and hydrophobic polymers is used to make the drug-containing matrix core. This is because it ensures that both highly and moderately water-soluble compounds get entrapped in the polymer’s corresponding matrices. Swellable polymers are preferred as they increase the hydrostatic pressure within the pump, but if the drug is highly water-soluble, then non – swellable polymers are used.
- Wicking agents – These are agents that have the ability to draw water into the porous network of the delivery device. They increase the contact area between the drug and the aqueous body fluids. Commonly used wicking agents include colloidal silicon dioxide, polyvinyl pyrrolidone ( PVP ), and sodium lauryl sulfate.
- Solubilizing agents are added when the drug used has low intrinsic water solubility. These agents increase the drug’s solubility by complexing with the drugs or inhibiting the drugs’ crystal formation. These include tween 20, polyethylene glycol ( PEG 8000 ), and triethyl citrate.
- Osmogens – Following penetration of the biological fluids into the pumps, they dissolve the osmogen and create an osmotic pressure within the pump. This pressure pushes the medicament outside the pump. Commonly used osmogens include potassium chloride, sodium chloride, and mannitol.
Surfactants – These agents help to maintain the integrity of the pump in the environment during the drug release period. They are added to the wall–forming material and regulating the different components’ surface energy to form a composite blend. Poly oxyethylenated glyceryl recinoleate, glyceryl laurate, and glycerol are commonly used surfactants.
- Plasticizers – Plasticizers or low molecular weight diluents are added to improve polymers’ film-forming characteristics. They act by changing the viscoelastic behavior of polymers used in the pumps. Modification of the polymers’ physical properties involves turning a hard and brittle polymer into a softer, more pliable material. This makes it more resistant to mechanical stress. Generally, around 0.001 to 50 parts of a plasticizer or a mixture of plasticizers are added to the polymers while manufacturing osmotic pumps—Eg. PEG 600, triacetin, triethyl phosphate, etc.
- Pore-forming agents – These agents cause the formation of the microporous membrane by its leaching activity during the operation of the pump within the body. The microporous membrane is formed in situ and is particularly beneficial for pumps that deliver poorly water-soluble drugs. Commonly used pore-forming agents include sodium chloride, calcium nitrate, sorbitol, and polyhydric alcohols.
Some of the commonly available osmotic pumps are :

Elementary osmotic pump ( EOP ) – This is the simplest type of osmotic pump in which drug from the core is released via the orifice in the non – extensible membrane following the increase in the volume of the osmogene due to the imbibition of water.
- Push – pull osmotic pumps ( PPOP ) – The structure of this device is similar to that of a bilayer coated tablet. The upper contains the drug in the polymeric matrix along with the osmotic agent and other excipients. The other layer, however, contains only the osmotic agent with other excipients. These layers are bound together and coated by a semipermeable membrane. Due to the osmotic attraction of the drug, water gets pulled into the upper layer first and then eventually ends up being pulled into the second layer ( nondrug layer ). This causes the second layer to expand and forces the drug via the delivery orifice in the pump.
- Sandwiched osmotic tablet ( SOT ) – In this type of pump, the polymeric push layer is sandwiched between two layers of the drug with a delivery orifice on each side. When exposed to the aqueous environment, the middle layer swells and causes the drug to be released from the opposite sides.
Other types of osmotic pumps available in the market are liquid-oral osmotic pumps and controlled porosity osmotic pumps.

Pingback: Implantable Drug Delivery System > PharmaCampus